- Journal Thesis
-
Cervicography and Pap co-testing had superior screening capacity and
cost-effectiveness in countries with a high prevalence of cervical cancer - European Journal of Obstetrics & Gynecology and Reproductive Biology
- Development and Validation of Novel Digitalized Cervicography system
- Reference: Obstetrics & Gynecology Science 2016
-
The Performance of Telecervicography for Detection of Pre-invasive and
Invasive Disease of the Uterine Cervix as an Adjunctive Test to Pap Smears - Reference: Contemporary Oncology 2016
-
Comparison of Single-, double-, and triple-combined testing,
including Pap test, HPV DNA test and cervicography,
as screening methods for the detection of uterine cervical cancer - Reference : Oncology Report 2013
-
The comparative evaluation of clinical screening in combined tests
[cytology(ThinPrep), HPV DNA test(Hybrid capture Ⅱ), cervicography]
for uterine cervical cancer - Reference : 2006 FIGO Report
Screening capacity and cost-effectiveness of the human papillomavirus test versus Cervicography as
an adjunctive test to Pap cytology to detect high-grade cervical dysplasia
|
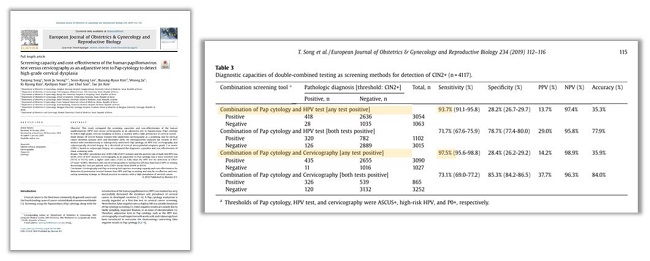
Among 4,117 women who simultaneously or subsequently underwent Pap cytology, HPV test, cervicography, and colposcopically directed biopsy, cervicography and Pap co-testing had superior screening capacity (sensitivity: 97.5% vs 93.7%) and cost-effectiveness (cost: $1,474 vs $1,135) for detection of pre-invasive cervical lesions than HPV and Pap co-testing and may be an effective and cost-saving screening strategy in clinical practice in countries with a high prevalence of cervical cancer. |
|
Ref) Taejong Song et al. Screening capacity and cost-effectiveness of the human papillomavirus test versus Cervicography as an adjunctive test to Pap cytology to detect high-grade cervical dysplasia. |
||||
| [European Journal of Obstetrics & Gynecology and Reproductive Biology 234 (2019) 112-116] |
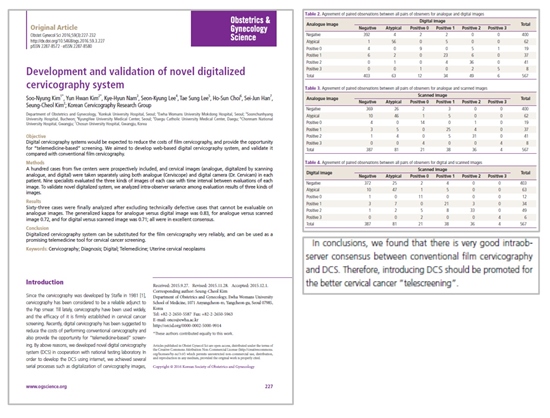
A study by Soo-Nyung kim et al (2016) included 100 patients to compare telemedicine-based cervico-graphy
(Digital cervicography system;DCS) with conventional film cervicography in the present study. In order to
validate the consensus rate of the telecervicography and conventional film cervicography, three methods;
analogue image, digitalized by scanning analogue image and digitalized image were evaluated by specialists.
Total 567 images were analyzed to assess intraobserver consensus and 91.9% (digital & analogue),
86.2% (analogue & scanned images), 86.1% (digital & scanned images) of diagnosis were identical with
kappa value 0.83, 0.72, 0.71 respectively; reflecting "almost perfect agreement" and "substantial agreement".
Thus, the conclusion is that there is very good intraobsever consensus between conventional film cervicography
and digital cervicography. This study is highly significant in that it is the very first study to validate the quality of
digital cervicography with that of conventional cervicography
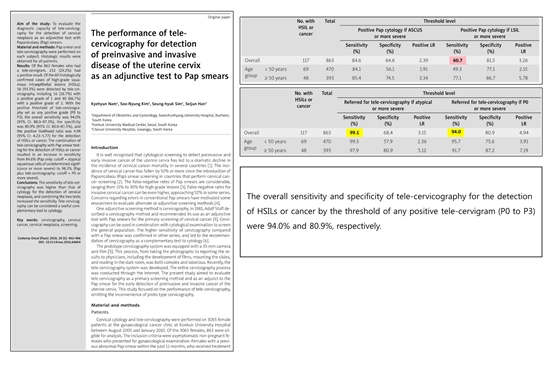
This paper was published in Contemporary Oncology in 2016 and 863 females have undergone tele-cervigram
and 252 with positive results were included in the study. It compares two cervical cancer screening method,
Pap smears and Telecervicography solely and combined at the same time. As Tele-cervicography and
Pap smear are done solely, the sensitivity finds out to be 94% and 60.7% each. The combination of
telecervico and Pap smear for the detection of HSILs or cancer resulted in an increase in sensitivity from 84.6%
(Pap only: ASC-US or more severe) to 98.3% (Pap plus Tele-cervicography over P0 or more severe).
In conclusion, Telecervicography is considered as a useful complementary tool to cytology for the accurate result.
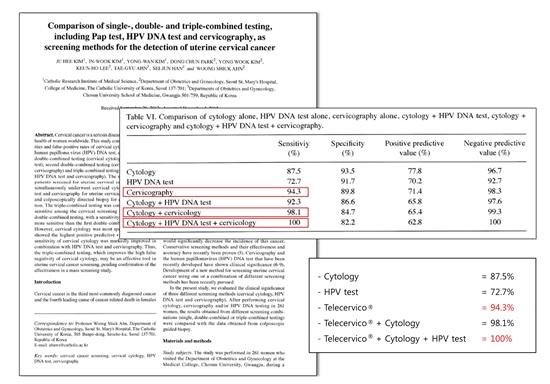
This paper was published in oncology report in 2013. The study included 261 patients screened for uterine
cervical cancer. All women simultaneously underwent cervical cytology, HPV DNA test and cervicography
for uterine cervical cancer screening and colposcopically directed biopsy for diagnostic evaluation.
The triple-combined testing was consistently the most sensitive among the cervical screening tests.
It compares three cervical cancer screening methods when implemented solely; cytology 87.5%,
HPV DNA test 72.7%, and cervicography 94.3% respectively. Cervicography has been concluded as the highest
sensitivity screening test as a sole test. Combining all three methods would improve the sensitivity up to 100%
which infer to extremely high accuracy in detection of cervical lesions.
A study by SJ Han et al (2006) included 252 patients who were underwent the biopsy among 829 patients who
had cervical cancer screening from Dec. 2001 to Sep. 2005. These 252 patients simultaneously underwent triple
combined test [cervical cytology(ThinPrep®), HPV DNA test (Hybrid capture Ⅱ®, Cervicography®(DCS®) and
colposcopically-directed biopsy on operation for diagnostic evaluation. The results shown in Table 2 confirmed
that the triple combined test [cervical cytology (ThinPrep®) + Cervicography®(DCS®) + HPV DNA test(Hybrid
capture Ⅱ ®)] showed a sensitivity of 96.0%, while double combined test [cervical cytology(ThinPrep®)+
Cervicography®(DCS®)] showed a sensitivity of 89.0%, the other double combined test [cervical cytology
(ThinPrep®)+ HPV DNA test (Hybrid capture®)] showed a sensitivity of 86.7%. Cervicography®(DCS®)
showed a specificity of 75.4% (highest among the single test), positive predictability of 89.8% (also highest).
The conclusion was that the sensitivity of cervical cytology was markedly improved by combination with the
Cervicography® (DCS®) and HPV DNA test.
| Table 2. | Comparison of ThinPrep® , HPV DNA test, Cervicography®(DCS®), ThinPrep® + HPV DNA test, ThinPrep® + Cervicography®(DCS®), ThinPrep® + HPV DNA test + Cervicography®(DCS®) |
| (n = 252 patients) | Sensitivity | Specificity | Positive predictive value |
Negative predictive value |
| ThinPrep® | 76.40% | 68.10% | 86.60% | 51.70% |
| HPV DNA test | 79.80% | 62.30% | 84.90% | 53.80% |
| Cervicography®(DCS®) | 81.00% | 75.40% | 89.80% | 59.80% |
| ThinPrep® +HPV DNA test | 86.70% | 52.90% | 83.00% | 60.00% |
| ThinPrep® +Cervicography®(DCS®) | 89.00% | 48.50% | 84.00% | 57.90% |
| ThinPrep®+ Cervicography®(DCS®)+ HPV DNA test |
96.00% | 35.40% | 80.20% | 76.70% |
| (Period:12/01/2001~09/30/2005) |

